SCIP Connect
Structure
Introduction
SCIP is the database for information on Substances of Concern In articles as such or in complex objects (Products) established under the 2018 revision to the Waste Framework Directive. From 5 January 2021, all companies in the EU are obligated to submit information into the SCIP database for any products they supply which include articles that contain REACH Candidate List substances above 0.1%, with possible subsequent enforcement by Member State regulatory authorities.
Reporting requirements apply to all companies in the supply chain including original article suppliers, suppliers of sub-assemblies and assemblies that contain affected articles, suppliers of finished products that contain affected articles, and resellers and distributors who supply finished products. The BOMcheck SCIP tools enable the easy management and submission of articles to the SCIP database.
Dashboard
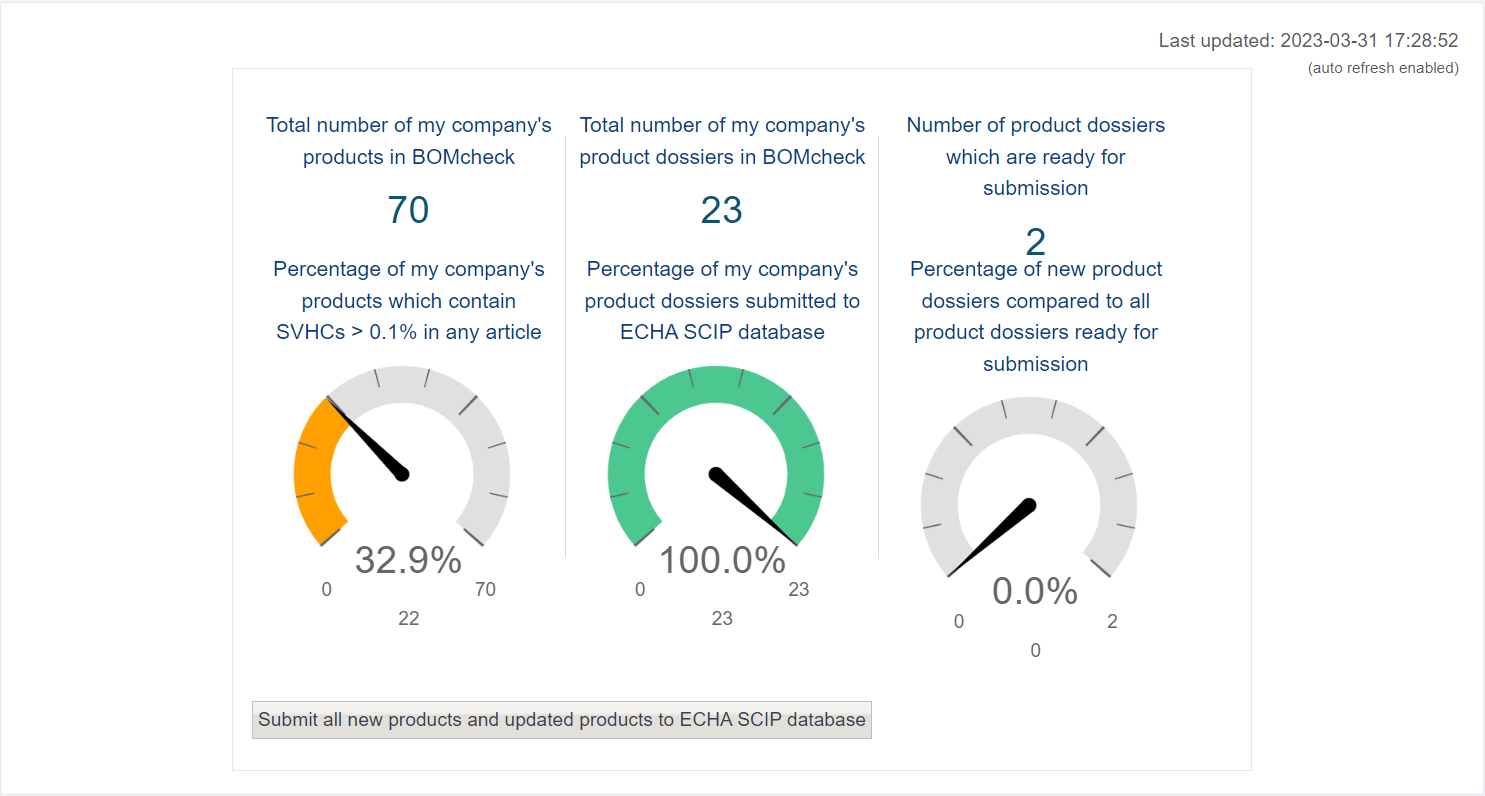
You can use the dashboard to view various metrics.
- Firstly, the number of a company’s products in BOMcheck and the percentage of them which contain SVHCs above 0.1 %
- The middle metric displays the number of product dossiers in BOMcheck, and the percentage submitted to the ECHA SCIP database. BOMcheck will create one every time it finds a product.
- The last metric displays the product dossiers which are ready for submission.
Lastly from the Dashboard page, you can click the button below to manually submit all your dossiers to the SCIP database.
Assess Data
The assess data tool lists all articles/complex objects requiring SCIP submission. Only articles which contain an SVHC above 0.1% are submitted. You can search for a specific part by name or number at the top of the page.
The last submission date indicates the date the article/complex object was submitted to the ECHA database. Clicking on “Submission report” will display the status of the submission, SCIP number and a URL to a report on the ECHA database. This will show the submission in ECHA’s portal confirming it was successful.
Take Note
“If SCIP submissions have received a successful validation response but aren’t showing in the SCIP database, this is likely due to a delay on ECHA’s side due to processing times and is out of BOMcheck’s control. “
Clicking on any part number will expand with further options such as full compliance details and the history of the parts.
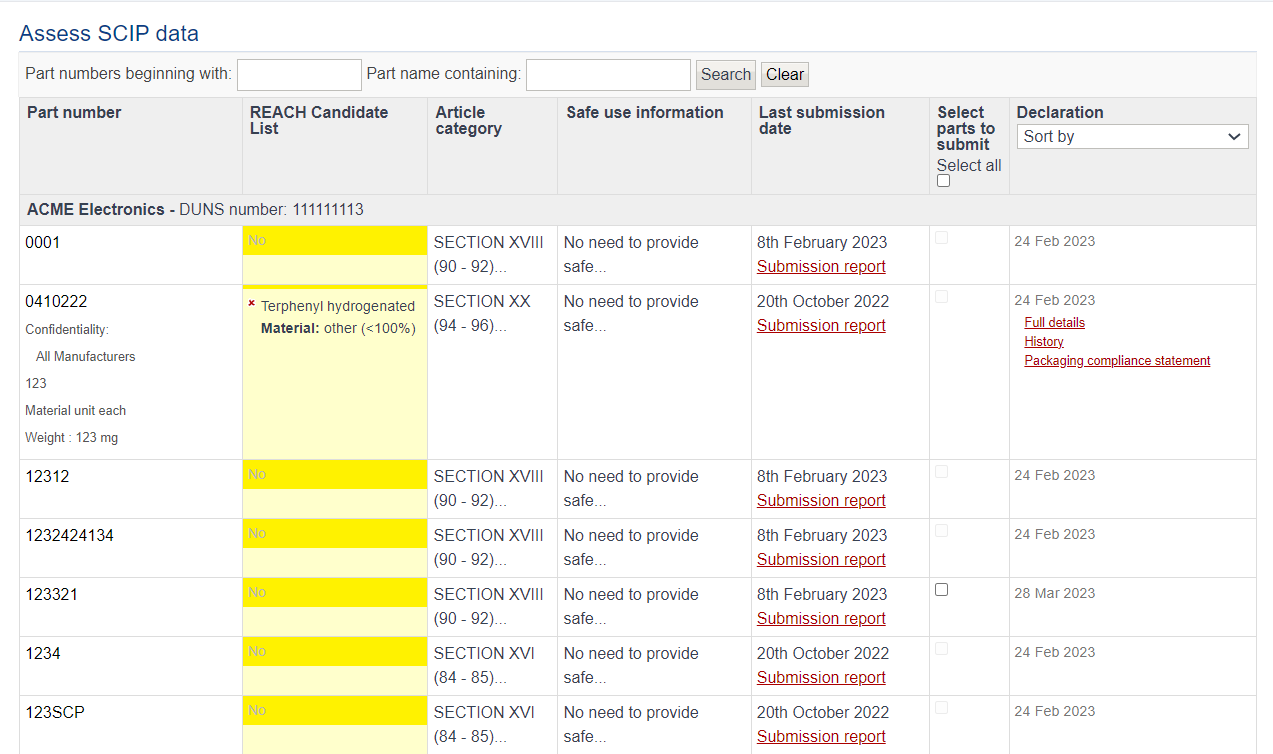
From the tool, you can also manually select individual parts you want to submit to the ECHA database,
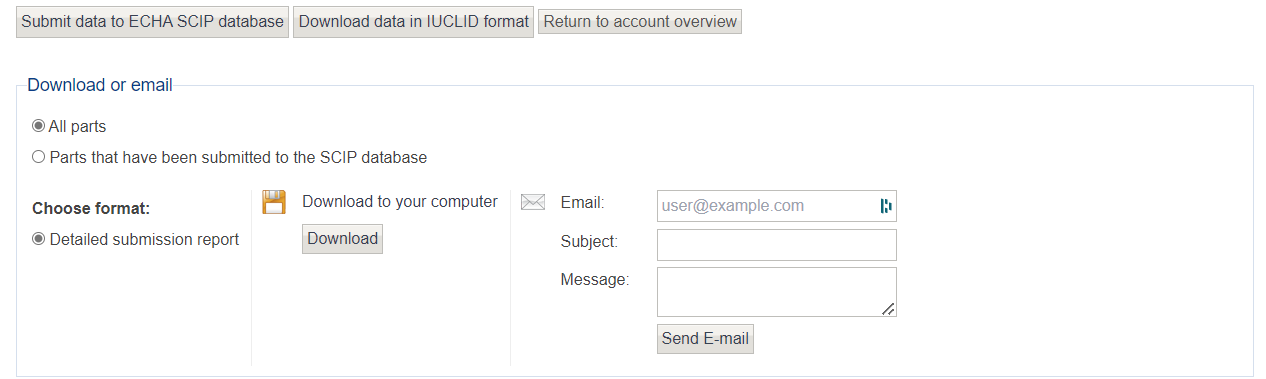
Lastly, you can download any data submitted to the ECHA database using the assess data tool.
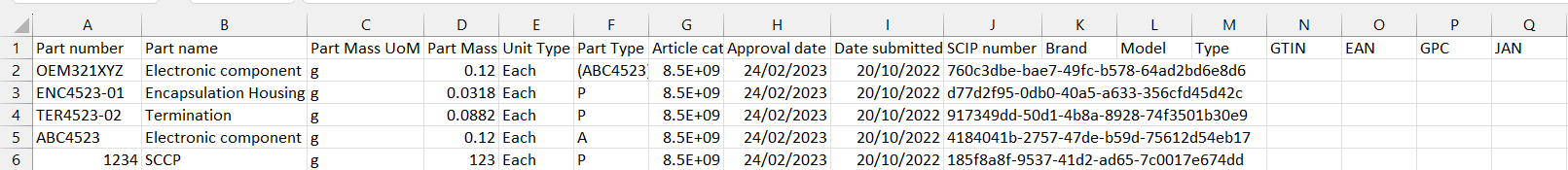
The submission report provides information on the part and the SCIP number in column J. It also provides columns for various labelling information which can be added to your submission. This is covered in the Add Identifier tool.
Add Identifiers
Barcodes, labels, catalogue numbers etc. are all important for consumer products. When you make a submission for a consumer product it should be easily searchable by the consumer.
Since information such as part numbers are not consumer friendly and are used internally, the add identifier tool in the BOMcheck SCIP module allows you to add identifiers such as barcodes and catalogue numbers to your product.
To do this you create a spreadsheet with the Part Number in Column 1 and the other identifiers in the subsequent columns. Once the information is added, save the file as a Unicode Txt file and upload it to BOMcheck. If you then download a submission report from the Assess Data tool for those part numbers, you will now see the various identifiers you uploaded included in that report.
Settings
You can choose between manual, automatic, and timed submissions to best suit your needs. When set on automatic, any new or updated article that contains an SVHC above 0.1% will be submitted to the SCIP database.
TIP
"We recommend that you start with your submission settings on manual until you are familiarised with the process. You should then change the submissions to automatic so BOMcheck will work in the background to make SCIP submissions for any affected articles”.